Why is glioblastoma so deadly? DelMar discusses the need for new cancer research and treatments for Brain Tumor Awareness Month
Glioblastoma is the most common form of primary brain cancer
VANCOUVER, Canada and MENLO PARK, Calif., May 1, 2014 /PRNewswire/ -- Jeffrey Bacha, president and CEO of DelMar Pharmaceuticals, Inc. (OTCQB: DMPI) ("DelMar Pharma" or "DelMar") discusses new clinical research and treatments in development for glioblastoma, the most common and severe form of brain cancer. Less than one out of three patients will survive two years after their diagnosis. DelMar is a cancer-focused company developing new therapies for patients with little to no treatment options.
To view the multimedia assets associated with this release, please click: http://www.multivu.com/mnr/7037951-delmar-pharmaceuticals-glioblastoma-brain-tumor-awareness-month
See the video of Mr. Bacha discussing the current clinical research and new therapeutic approaches for treating refractory glioblastoma.
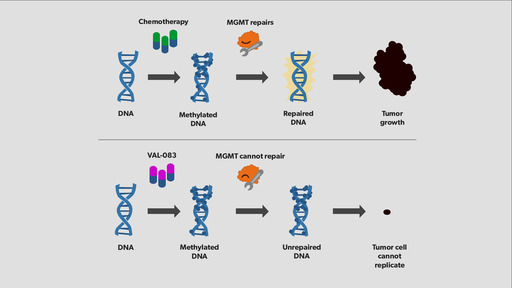
Learn more about what causes chemotherapy resistance and how new therapies in development work to better fight glioblastoma.
Today's approved front-line chemotherapies to treat glioblastoma work through the same mechanism of action that can be counteracted by an enzyme called MGMT. DelMar's first drug in development, VAL-083, works differently to kill cancer cells and is unaffected by MGMT, making it a potential first-in-class treatment for glioblastoma multiforme (GBM).
Quotes:
Jeffrey Bacha, president and CEO of DelMar Pharma, "We must continue to raise awareness about glioblastoma because more research and new treatment options can make a difference in this deadly disease."
"We are testing VAL-083 in refractory glioblastoma, where there is the greatest need and greatest potential impact, and we are running our clinical studies as quickly as possible to get this promising drug into the hands of physicians to treat the patients who need it the most."
Key Facts:
- More than 12,000 people are diagnosed with glioblastoma each year in the United States.
- Approximately two out of three glioblastoma patients will fail today's approved therapies.
- Many patients develop resistance to the front-line therapy, Temodar®, because of an enzyme known as MGMT.
- VAL-083 acts through a mechanism independent of MGMT.
- Historical clinical trials and interim clinical trial results presented at AACR and ASCO suggest VAL-083 is safe and well tolerated by patients at doses tested to date.
- Studies by the National Cancer Institute and DelMar have shown VAL-083 to have activity against a range of cancers, including glioblastoma.
- New immune-based therapeutic approaches, such as cancer vaccines, gene therapies and viral therapies, such as modified polioviruses, are often combined with chemotherapy, so developing new chemotherapies, like VAL-083, is essential.
- DelMar is conducting a Phase I/II clinical trial for VAL-083 at UC San Francisco and the Sarah Cannon Research Institute in Nashville, Tennessee and Sarasota, Florida.
- VAL-083 has received orphan drug designation in Europe and the United States.
Additional Information:
- Brain Tumor Awareness Month
- About Glioblastoma Multiforme (GBM)
- About VAL-083
- Clinical Trials for VAL-083
About DelMar Pharmaceuticals
DelMar Pharmaceuticals was founded in 2010 to develop and commercialize proven cancer therapies in new orphan drug indications where patients are failing modern targeted or biologic treatments. The Company's lead asset, VAL-083, is currently undergoing clinical trials in the United States as a potential treatment for refractory glioblastoma multiforme (GBM), the most common and aggressive form of brain cancer. VAL-083 benefits from extensive clinical research sponsored by the U.S. National Cancer Institute, and is currently approved for the treatment of chronic myelogenous leukemia (CML) and lung cancer in China. Published pre-clinical and clinical data suggest that VAL-083 may be active against a range of tumor types via a novel mechanism of action.
For more information, please visit www.delmarpharma.com or stay up-to-date on Twitter @delmarpharma and Facebook.com/delmarpharma
Safe Harbor Statement
Any statements contained in this press release that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. Any forward-looking statements contained herein are based on current expectations, but are subject to a number of risks and uncertainties. The factors that could cause actual future results to differ materially from current expectations include, but are not limited to, risks and uncertainties relating to the Company's ability to develop, market and sell products based on its technology; the expected benefits and efficacy of the Company's products and technology; the availability of substantial additional funding for the Company to continue its operations and to conduct research and development, clinical studies and future product commercialization; and, the Company's business, research, product development, regulatory approval, marketing and distribution plans and strategies. These and other factorsare identified and described in more detail in our filings with the SEC, including, our current reports on Form 8-K. We do not undertake to update these forward-looking statements made by us.



To view the multimedia assets associated with this release, please click: http://www.multivu.com/mnr/7037951-delmar-pharmaceuticals-glioblastoma-brain-tumor-awareness-month
SOURCE DelMar Pharmaceuticals, Inc.
Released May 1, 2014